ABSTRACT
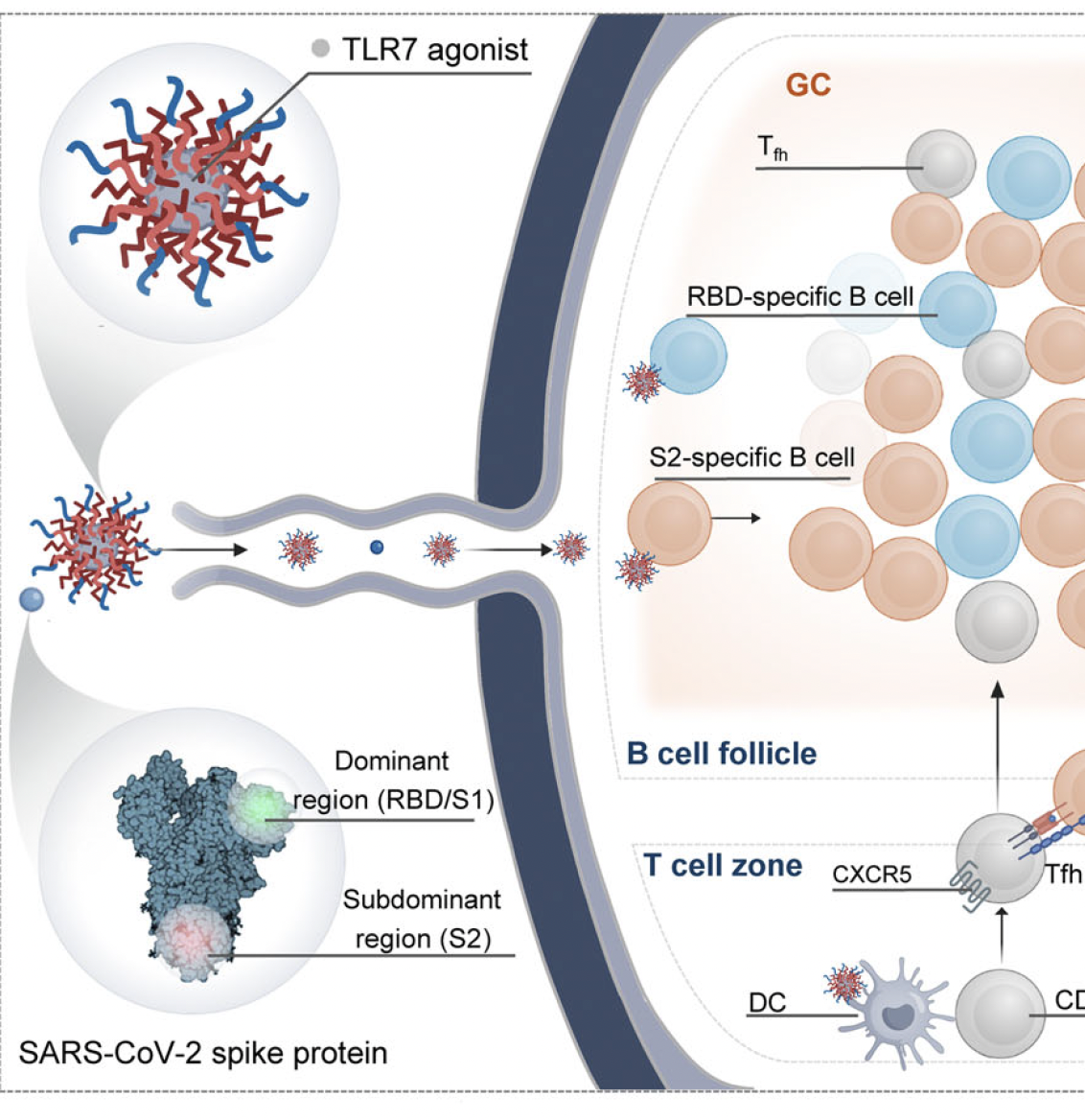
Current SARS-CoV-2 vaccines primarily elicit antibodies targeting the variable receptor-binding domain in the S1 subunit of the spike protein, resulting in limited cross-reactivity and short-lived immunity against emerging variants. The conserved S2 subunit presents a promising vaccine target for broad and durable protection, but the immunodominance in vaccine-induced germinal center (GC) responses hinders effective antibody generation against S2. Here, a polymeric toll-like receptor 7 agonist nanoparticle (TLR7-NP) adjuvant is reported, well designed to enhance lymph node targeting and more efficiently activate S2-specific B cells. When combined with Alum-adsorbed SARS-CoV-2 HexaPro spike protein, TLR7-NP promotes early GC recruitment of S2-specific B cells and overcomes the immunodominance, leading to early and robust S2-specific antibody responses. Compared to conventional TLR7-Alum adjuvanted subunit vaccine and clinically used SARS-CoV-2 mRNA vaccine, TLR7-NP adjuvant induces stronger humoral immune responses across sarbecoviruses and betacoronaviruses and promotes long-lived plasma cell and memory B cell formation. These findings present a direct B cell-activating adjuvant approach for effective pan-coronavirus vaccine development.